This is the third in our series of DCA data analysis updates.
When we presented our first DCA observational data analysis in December 2007, our intent was to share our knowledge of treating patients with DCA and to show the potential of DCA as a viable cancer treatment option outside the laboratory in the real world for the first time. With our second data update in March 2008, we showed that our DCA treatments results were consistent with our earlier analysis and further solidified our views of using DCA in cancer treatment. For our next update, since our overall DCA treatment data showed similar results as before, we decided to present DCA case reports instead of a complete data update. Since then we have received numerous requests and enquiries about another data update and are happy to present our most recent findings here.
It has taken us longer than we anticipated compiling this report. We were hoping to provide an update in May 2009, but our regular clinic work (treating cancer patients) took precedence over data analysis and updates. Since we are not conducting a clinical trial and our main focus is patient care and providing novel care options, the pure data collection and analysis aspect of our work sometimes has to wait. As always we welcome input from our patients to better serve your needs.
Just like our previous updates, the data presented below is not from a clinical trial. It is based on our observations of the patients we have treated so far and does not meet the rigorous criteria employed in clinical research. It may not be generalizable. We are sharing this to further patient care and improve medical understanding and knowledge regarding DCA as a cancer treatment.
Patient Demographics
At Medicor Cancer Centres, as of April 2009, we have treated 347 patients with DCA. Most of these patients have exhausted conventional treatment options. The proportion of males and females treated was 48% males to 52% females.
Patients treated range in age from 2 years to 90 years. The majority of patients (96 patients, or 29%) are 50- 59 years old, followed by patients who fall in the 60-69 year age group (79 patients, or 24%).
Age Distribution of Patients Treated with DCA

The site of primary cancer in the 347 patients is presented below.
Site of Primary Cancer in Patients Treated with DCA
| Site of Cancer |
Number of Patients Treated |
% of Patients Treated |
| Lung |
68 |
20% |
| Brain |
63 |
18% |
| Colon |
44 |
13% |
| Breast |
30 |
9% |
| Pancreas |
19 |
6% |
| Ovary |
13 |
4% |
| Prostate |
11 |
3% |
| Lymphatics/lymphoid tissue |
9 |
2% |
| Stomach, Cervix |
8 (each)
|
2% (each)
|
| Esophagus, Skin |
6 (each)
|
2% (each)
|
| Bladder, Kidney, Gall bladder |
4 (each)
|
1% (each)
|
| Salivary Gland (adenoid cystic) |
3
|
1%
|
| Bile duct, Blood (leukemia), |
2 (each)
|
1% (each)
|
| Endometrium, Head, Lymphoma, |
2 (each)
|
1% (each)
|
| Oropharynx, Sarcoma |
2 (each)
|
1% (each)
|
| Carcinosarcoma, Eye, GI Stromal, |
1 (each)
|
0.5% (each)
|
| Hard Palate, Leiomyosacrcoma, Liver, |
1 (each)
|
0.5% (each)
|
| Leiomyosacrcoma, Liver, Mediastinum, |
1 (each)
|
0.5% (each) |
| Pelvis, Rectum, Retroperitonium, Sinus, |
1 (each)
|
0.5% (each) |
| Spinal Cord, Spine, Thyroid, Ureter, |
1 (each)
|
0.5% (each)
|
| Urethra, Vagina |
1 (each)
|
0.5% (each)
|
| Unknown Primary |
15
|
4%
|
Patient Responses
Our treatment regimen generally consists of DCA in a 2 week on / 1 week off cycle which is modified based on side effects (e.g. ranges from 1 week to 3 weeks of DCA followed by a rest period). In children we sometimes offer continuous DCA treatment. Typical doses are in the range of 20-25mg/kg/day for adults and 25-50mg/kg/day for children. For elderly patients or those with complicating factors, we may use less than 20mg/kg/day. The reason that we favour cyclic DCA treatment is that it allows us to use higher doses and minimize side effects at the same time by having a period of time where DCA can be cleared from the body. We believe a dose-response relationship exists with DCA (higher doses = better response), therefore cyclic treatment may provide a higher response rate while preventing the drug from being discontinued due to adverse events. It also allows us to clearly determine which side effects are from DCA and which are due to other causes (like the cancer itself). This can be invaluable in determining if DCA should be stopped, or may be safely continued.
We are treating more patients with combination therapy (DCA + 1 or 2 other drugs) and feel more confident now that DCA works better in combination with other agents than by itself over a prolonged time period.
All of our patients understand that a positive to DCA treatment is not a guarantee and just like most chemotherapies, the individual response varies. We encourage discussion of treatment options, known side effects, monitoring protocol, and the uncertainty and paucity of knowledge of the long term effects of DCA in cancer treatment.
We continue to use our criteria of evaluating patient responses after a minimum of 4 weeks from start of treatment for the following analyses.
Based on this criterion, as of April 2009, we were able to evaluate 179 (52%) of our total (347) patients treated. The reasons for not evaluating the 168 patients are as follows:
-
- 80 (48%) stopped DCA after < 4 weeks for various reasons. These were mostly telemedicine patients who were lost to follow-up. We introduced telemedicine for convenience of patients who do not live locally, are unable to travel, and cannot find a local doctor to prescribe DCA. While convenient for the patient it does not replace seeing the patients in person. Patients opting for telemedicine care are advised to have a local doctor to consult and follow up with in addition to Medicor doctors. We also see more patients who we lose touch with and are unable to follow up regularly. Some of these patients may have responded to DCA and continued their treatment beyond 4 weeks from another source while others may not have responded and have abandoned DCA treatment. While we have come to know of such patients through other sources, if we cannot establish contact with them ourselves (after at least three attempts) to verify their progress, we classify them in this category.
- 74 (44%) of patients died within 4 weeks of starting DCA due to reasons not related to the treatment itself. (We do not deny patients access to safe treatment based on stage of illness and sometimes patients come to us at a very advanced disease stage.)
- 14 (8%) had started treatment recently (< 4 weeks) in April 2009 and we have not included them in this analysis. As of this date, these patients may be evaluable but our cut off date for this analysis was April 2009 for logistical reasons.
Patient Evaluation Status 4 Weeks
after start of DCA Treatment

Responses in Patients After at Least Four Weeks of DCA Treatment
Based on our evaluation criteria, we have evaluated 179 patients who took DCA for at least 4 weeks. We have chosen the 4 week mark for our analyses to allow time for DCA to work, and to eliminate the placebo effect (which can typically last for 1-2 weeks). Please be advised that this is not a standard criterion and other people may choose to use different criteria in their research. In fact, the 4 week cutoff likely underestimates true DCA response. We have seen patients improve only after 8 weeks of DCA treatment in some cases.
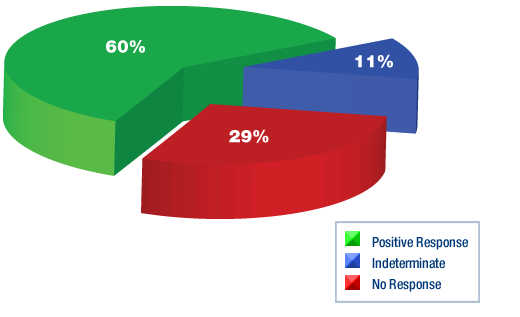
In these 179 patients we have observed the following responses to DCA:
-
- 106 patients (60%) showed a positive response to DCA Similar to our previous analyses, we have divided the positive responses into the following five categories based on the degree of clinical benefit. The categories below are not mutually exclusive since patients may have had a combination of positive responses while on DCA. For example a patient who had a reduction in tumour size and symptomatic improvement is counted in both categories 1 and 4. For this reason, the numbers below do not add up to 106. This presents an overall picture of the responses to DCA.
- Category 1: Reduction in tumour size/complete tumour regression. Seen in 21 patients (12%). We are very excited to report that in 2 patients (1%) we have seen complete remission of metastatic cancer. The other 19 patients (11%) had measurable tumour reduction which was demonstrated by imaging studies and/or direct tumour measurement.
- Category 2: Reduction in tumour markers. Seen in 16 patients (9%). These patients had a reduction in markers such as CEA, CA-125, CA19-9, CA15-3 or AFP. These types of blood markers are typically used to monitor certain cancers such as colon, ovarian, prostate, pancreatic, lung, liver, or breast.
- Category 3: Improvement in blood tests. Seen in 12 patients (7%). These included improvements in hemoglobin, liver enzymes, albumin, and other tests indicating reduced tissue damage or reduced cancer activity that could not be explained by other events or medications.
- Category 4: Symptomatic improvement. Reported in 52 patients (29%). This included significant pain reduction, relief of bowel obstruction, weight gain, improved appetite and improved energy level. These improvements were sustained for a period of more than 4 weeks, thus making it unlikely to be a result of placebo effect.
- Category 5: Disease stabilization. Seen in 61 patients (34%). In these patients, there was no evidence of cancer progression while taking DCA, where progression would otherwise have been expected.
To complete the picture, the following data shows only the best response of each of the 106 patients who had a positive response. We have chosen to define the responses in decreasing level from category 1 to 5 with category 1 (reduction in tumour size) being the best response and category 5 (disease stabilization) being the lowest level of positive response to DCA. The data is categorized as above but here the categories are mutually exclusive with a total of 106 responses. This is unlike the data we have presented previously and therefore is not comparable to our earlier analyses.
-
- Category 1: Reduction in tumour size. Seen in 21 patients (12%) including 2 patients (1%) with complete tumour regression/complete remission. These patients may also have experienced improved blood tests, tumour markers or symptoms, but are not counted in those categories.
- Category 2: Reduction in tumour markers. Seen in 14 patients (8%). These patients may also have experienced improved blood tests, improvements in symptoms or disease stabilization, but are not counted in those categories.
- Category 3: Improvement in blood tests. Seen in 6 patients (3%). These patients may also have experienced improved symptoms or disease stabilization, but are not counted in those categories.
- Category 4: Symptomatic improvement. Reported in 33 patients (18%). These patients may also have experienced disease stabilization, but are not counted in that category.
- Category 5: Disease stabilization. Seen in 32 patients (19%).
- The total of Categories 1-5 is still a 60% positive response rate.
- In 20 patients (11%), there was no way to determine if the cancer responded or progressed. These patients had no readily measurable tumour, no relevant tumour markers, and blood tests with no major abnormalities.
- In 53 patients (29%), there was no response to DCA treatment. In these patients, the cancer progressed despite DCA treatment, including increased doses.
Patient Responses to DCA Treatment
Type of Positive Response to DCA Treatment

Site Specific DCA Response Rates
The site of primary cancer in the 179 patients who were evaluated after at least 4 weeks from start of DCA is as follows:
| Site of Primary Cancer |
Number of Patients |
Percentage of Patients |
| Brain |
38 |
21% |
| Lung |
37 |
21% |
| Colon |
22 |
12% |
| Breast |
16 |
9% |
| Ovary |
10 |
6% |
| Pancreas |
7 |
4% |
| Lymphatics/lymphoid tissue, Cervix (each) |
6 (each)
|
3% (each)
|
| Prostate, Skin (each) |
4 (each)
|
2% (each)
|
| Stomach |
3
|
2%
|
| Head & neck, Bladder, (each) |
2 (each)
|
1% (each)
|
| Kidney, Gall Bladder (each) |
2 (each) |
1% (each) |
| Salivary Gland, Blood (leukemia), GI Stromal, |
1 (each) |
0.5% (each) |
| Endometrium, Esophagus, Hard Palate, |
1 (each)
|
0.5% (each)
|
| Lymphoma, Mediastinum, Pelvis, Sarcoma, |
1 (each)
|
0.5% (each)
|
| Spine, Ureter, Vagina (each) |
1 (each)
|
0.5% (each)
|
| Unknown Primary |
5
|
3%
|
Brain Cancer: The 38 patients were subdivided into gliomas (37) and other cancers (1).
Out of the 37 patients with gliomas, 23 (62%) showed a positive response, 5 (14%) were indeterminate, and 9 (24%) had no response to DCA treatment.
The duration of treatment was from 4 to 68 weeks.
Lung Cancer: Of the 37 lung cancer patients, 19 had non- small cell carcinoma, 4 had mesothelioma, 3 had non-small cell carcinoma and in 11 patients the type of cancer was unknown. It is most likely that the 11 patients with unknown type of cancer had a non small cell carcinoma but it is not confirmed.
The results for the 19 non small cell carcinoma patients have been reported previously (10 (52%) showed a positive response, 3 (16%) were indeterminate and 6 (32%) had no response to DCA treatment). The other numbers are too small for meaningful interpretation. The maximum duration of treatment was 24 weeks.
Colon Cancer: Of the 22 colon cancer patients treated, 17 (77%) showed a positive response, 4 (18%) did not respond and the response in 1 patient (5%) was indeterminate. The duration of treatment ranged from 4 – 36 weeks.
Breast Cancer: Twelve (75%) of the 16 patients responded positively to treatment while 4 (25%) did not respond. The maximum duration of treatment was 44 weeks.
Ovarian Cancer: Out of the 10 patient treated, 7 (70%) responded to treatment while 3 (30%) did not respond. The treatment was continued for up to 40 weeks.
Other cancers: While the numbers are too small for any meaningful interpretation, we have seen results similar to the above. Since these numbers are not meaningful, they will not be listed.
Duration of treatment
The duration of treatment in the 179 evaluable patients ranged from, 4 weeks to 68 weeks.
Duration of DCA Treatment in Patients
Who Were Evaluated After at Least 4 Weeks
As of April 2009, 159 (89%) patients had stopped treatment with DCA while 20 (11%) were continuing treatment. This is not a static number and you can get an idea of the current numbers from the Live Update page. The reasons for stopping treatment in the 159 patients are presented below.
Reasons for Stopping DCA Treatment
| Duration in Weeks |
Number of Patients |
Percent |
| Brain |
38 |
21% |
| Up to 6 weeks |
52 |
29% |
| 7-12 weeks |
93 |
52% |
| 13-24 weeks |
21 |
12% |
| 25-36 weeks |
9 |
5% |
| 37+ week |
4 |
2% |
| Total |
179 |
100% |
| Site of Primary Cancer |
Number of Patients |
Percentage of Patients |
| Patient died |
46 |
29% |
| Not effective |
40 |
25% |
| Lost to follow up |
25 |
16% |
| Side effects |
18 |
11% |
| Started other treatment |
13 |
8% |
| Other non-medical reasons |
10 |
6% |
| Other medical reasons (incl hospital refused administration. |
6 |
4% |
| Unknown |
1 |
1% |
| Total |
159 |
100% |
Side Effects
We continue to hold the view that DCA is a relatively safe cancer treatment (especially compared to toxic treatment like chemotherapy) and that use of supplements such as alpha lipoic acid and benfotiamine (vitamin B1) are helpful in managing the neurological side effects. In some patients side effects are apparent quite soon while others seem to do quite well from several months to nearly 2 years of treatment. While most side effects seem reversible, we do not know what the long term effects will be (> 2 years). There is no medication which is known to completely prevent DCA-related neurological side effects such as neuropathy.
Out of the 179 patients who were evaluable for response, the frequency of reported side effects is as follows:
| Side Effect |
Frequency |
Percent |
| None |
78 |
44% |
| Fatigue |
26 |
15% |
| Numbness |
20 |
11% |
| Confusion |
16 |
9% |
| Tremor |
14 |
8% |
| Sedation |
3 |
2% |
| Hallucination |
3 |
2% |
| Leg Weakness |
2 |
1% |
| Paresthesia |
2 |
1% |
| Heartburn |
2 |
1% |
| Agitation |
0 |
0.5% |
| Anxiety |
1 |
0.5% |
| Sweating |
1 |
0.5% |
| Unknown |
19 |
11% |
Comments and Opinions
Our observational DCA data continues to show consistent rates of response and mild side effects confirming that DCA has the potential to be a useful part of a cancer treatment plan. We are receiving comments from many physicians indicating increased levels of interest in DCA and increased willingness to support their patients who wish to use DCA. We are also receiving positive feedback from physicians around the world who have used DCA and noted positive results.
However, the most common physician response is that DCA is unproven as a cancer treatment, and physicians refuse to prescribe it on that basis. To call a treatment “proven†is not an absolute definition. Rather, there are various levels of proof that a treatment is effective. DCA has not met the highest level of proof namely a phase 3 multi-centre randomized double-blind placebo-controlled crossover trial. However, we have established DCA’s effectiveness as a cancer treatment by a lower level of evidence.Proof of Treatment EffectivenessThere are many ways to establish whether a disease treatment is effective or not. All these methods have their limitations. The only method that constitutes 100% proof that a treatment is effective is to treat a patient with a drug and observe the response, then go back in time, withdraw the drug and observe the response (and change nothing else). Clearly this is impossible, so we must rely on methods that are practical and possible.The most common methods are listed in order of decreasing strength of evidence:
- Randomized prospective multi-centre double-blind placebo-controlled crossover clinical trial. This is the best method we have to assess the effectiveness of a drug treatment. A group of patients is randomly assigned to take a treatment or a placebo, where neither the treating physicians nor the patients are aware of what they are receiving. At some point, the patients on placebo are switched with those on the real treatment and observation is continued. The trial is conducted at several research centres in different countries to enroll large numbers of patients and eliminate bias that could occur at a single centre. This method also attempts to circumvent the problem that we cannot go back in time, by crossing over patients from placebo to actual treatment (or the reverse).There are presently no such trials of DCA. For various ethical and financial reasons, it may not be possible to ever conduct such a DCA trial.
- Lesser clinical trials such as open label trials (everyone knows what the patients are getting), non placebo-controlled trials, small trials (e.g. with 10′s of patients rather than 100′s or 1000′s) and trials conducted at only 1 research centre.All of the 3 the current DCA clinical trials are small, single centre, open label (patients know what they are getting) and non-placebo controlled (for ethical reasons). This means they provide much weaker evidence than the best clinical trials. However they are still useful.
- Observational studies such as case-control studies, cohort studies and case series. These studies are based on observation of patients receiving treatment without providing a placebo group and without the more strict treatment criteria of a clinical trial. Medicor’s DCA Observational Data falls within this category.
- Case Reports. These are detailed reports of patients treated with a certain drug, prepared by their medical teams. Case reports carefully prepared by medical experts can be strongly suggestive that a treatment given to the patient is effective (especially if the patient had disease progression prior to the treatment, followed by stability or regression during treatment). Medicor has presented several case reports on this website.
- In vivo/in vitro lab research (e.g. treatment of rats implanted with human cancer cells or exposure of cancer cells to a new drug in a test tube). This type of study strongly suggests that a treatment may be effective, but requires human study to confirm. The original DCA cancer research published in Cancer Cell in Jan 2007 falls into this category.
- Testimonials. These are reports from patients indicating that their treatments were effective. Unfortunately, testimonials are often totally inaccurate because patients do not typically have the medical knowledge to determine if their treatment is effective, and to what extent (e.g. if they are taking more than one drug, or had a recent treatment that is still working when they start something new). Naturally, patients are also often guided by emotion, rather than scientific evidence. Testimonials are also used to sell various treatment drugs or devices for profit by carefully selecting and displaying a small number of patients who benefited from a treatment, with no mention of the large number who did not benefit. For this reason, use of patient testimonials is banned in advertising by physicians in Ontario. Medicor does not use testimonials at all.
Based on our observational data we believe that:
- DCA can be an effective component of a cancer treatment program.
- DCA is a viable option for patients who have exhausted conventional therapies.
- DCA is effective against many different types of cancers.
- DCA is relatively safe but has specific side effects and should be used under medical supervision only.
- Response to DCA is variable like any other medical cancer treatment
- DCA is more effective in healthier patients than those with advanced stage disease
- DCA can interact favourably or negatively with chemotherapy is some patients. This is hard to predict unless a chemosensitivity test like ChemoFit is performed.
- Duration of DCA treatment depends on individual patient. Limiting factors maybe loss of effectiveness or side effects.
- DCA maybe more effective and tolerable in children, but like adults, children may experience hallucinations or emotional changes due to DCA that resolve when the drug is stopped
We have noted that DCA by itself does not appear to be as effective (or curative) compared to combination therapy. When we initially started DCA therapy, most patients took DCA by itself. We observed that in patients who showed a positive response initially, DCA had to be stopped either due to side effects or because it started to lose its effectiveness. By adding supplements like alpha liopic acid, adjusting the dose and having a non-continuous DCA regimen we have managed to reduce the side effects to a more tolerable level. While patients started experiencing more tolerable and reversible side effects, the loss of effectiveness of DCA was troubling. Change in dose was not able to reverse this effect for long leading us to discontinue DCA treatment. However, when we started combining DCA with other treatments like TM therapy, we found that treatment could be continued for much longer and was more effective.
We have now started using DCA more in combination with other treatments (e.g. chemotherapy, TM, ribavirin, radiation therapy etc.) and have observed more favorable responses. For combination with chemotherapy we strongly recommend a ChemoFit test to determine effectiveness. While there are no general criteria to predict if patients will respond to DCA or a combination therapy with chemo, a ChemoFit test can provide very sensitive and specific response information for the individual patient.Our two cases of complete remission both took combination therapy.
A potential limitation of our data is the combination of DCA with other drugs. Our data asnd analyses does not account for this confounding factor. Many of our newer patients take combination treatments. Confounding is always a consideration in any clinical trial and different methods are used to minimize it. In our case, we acknowledge that our responses may not be purely DCA because of this confounding factor of combination therapy. We aim to treat patients with the best possible treatment options and unlike pure research, we do not stop other treatments which may confound our results. When we started our data collection, we did not anticipate this extent of analysis.
In spite of this potential confounding, our earlier data analyses (which were done before most combination therapies) have shown similar results. This gives us confidence in the accuracy of our results. In our previous analyses, our duration of treatment did not last long due to loss of DCA effectiveness whereas now we can continue longer with combination therapies. Nevertheless this potential limitation of our data should be kept in mind while viewing our results which are not from a clinical trial.
We have not previously acknowledged the psychological aspects of DCA treatment or quality of life enhancements. Subjective responses are always hard to report and categorize and we have based all our evaluations on objective and measurable criteria.
Our review of the subjective aspects of DCA treatment indicate that there is a very small number of patients who are unhappy about trying DCA, mainly because they experience significant side effects (such as neuropathy with burning pain). However, a majority of patients and their families are highly satisfied and uplifted by DCA treatment. The main reason for this is likely the fact that DCA substantially changes the outlook of patients who are deemed end stage palliative. Since DCA is a gentle cancer treatment with scientific merit, patients hope for survival or life extension is restored without fear of severe side effects (such as vomiting, diarrhea, hair loss, immune suppression/infection or other loss of quality of life that accompanies treatments like chemo). Even compared to approved targeted non-chemo treatments, DCA has fewer side effects, and does not appear to results in severe side effects like internal hemorrhage.
This subjective aspect of DCA treatment is invaluable and cannot be discounted.These statements are the opinions of Medicor physicians. They are based on existing research and our experience and observations in over 400 cancer patients treated with DCA. They should not be used as a guide to self medicate and the results may not be generalizable. We hope this information is helpful to physicians around the world who are interested in using DCA to treat their cancer patients.
For questions or comments regarding this page please email hkhan@medicorcancer.com